Small molecule based treatments
Two images often come to mind with small molecule drug discovery: a botanist finding a rare plant that contains some wonderful new drug or a chemist synthesizing a fantastical molecule. The combination of these two scenarios is actually closer to the truth. Combinatorial chemistry, as the name suggests, is a mix-and-match approach to chemical synthesis, allowing the production of millions of different products. Generally considered to have been inducted in the 1980s by Mario Geysen, "combichem" has dramatically accelerated drug development, but as a standalone approach, it has generated very few de novo drug molecules. On the other hand, products isolated from the environment that were produced by nature, "natural products", have been the source of many drugs. Once a candidate drug has been discovered, combichem is often used to improve the drugs efficacy. In fact, as of 2012, 68% of small molecule anticancer drugs were natural products or derived from natural products.
Unlike antibodies, small molecule therapeutics can be targeted with both intracellularly or extracellularly. For example, when a tumor reaches 1-2 mm in diameter, they begin to develop their own vasculature to provide enough nutrients for the tumor to continue to grow. Small molecule inhibitors can target vascular endothelial growth factor (VEGF), which is expressed on the cell surface of cells and promotes vascularization. Monoclonal antibodies (mAbs) act directly on VEGF, blocking the receptor to prevent vascularization. Small molecules acting as tyrosine kinase inhibitors act internally, blocking the downstream signaling of the receptor within the cell.
Antibody-based treatments
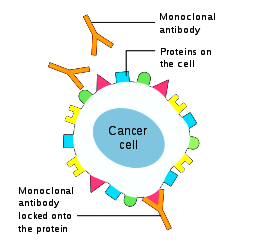
Over the past 20 years, antibody-based treatment has been one of the most successful strategies for treating cancer. As of 2012, there were at least 12 FDA approved monoclonal antibodies for the treatment of solid tumor and blood cancers. Unlike small molecules that can effect change intracellularly or extracellularly, antibody based treatment are based on cell surface antigens. Based on astounding work describing cancer cells, a detailed understanding of the cell surface receptors of cancer cells is growing, and aided by bioinformatics, could lead to a full picture of the "cancer surface-ome". Cancer cells often have antigens that are mutated or overexpressed compared to normal cells, creating ideal targets for antibody fragments. Selective expression in cancer cells compared to normal cells, which is sometimes considered a "magic bullet", has not yet been found.
The killing of tumor cells is done through direct action of the antibody such as blocking a growth-factor receptor, affecting the tumor vasculature, recruiting immune killer cells, or payload delivery (antibody-drug conjugates described below).
Two images often come to mind with small molecule drug discovery: a botanist finding a rare plant that contains some wonderful new drug or a chemist synthesizing a fantastical molecule. The combination of these two scenarios is actually closer to the truth. Combinatorial chemistry, as the name suggests, is a mix-and-match approach to chemical synthesis, allowing the production of millions of different products. Generally considered to have been inducted in the 1980s by Mario Geysen, "combichem" has dramatically accelerated drug development, but as a standalone approach, it has generated very few de novo drug molecules. On the other hand, products isolated from the environment that were produced by nature, "natural products", have been the source of many drugs. Once a candidate drug has been discovered, combichem is often used to improve the drugs efficacy. In fact, as of 2012, 68% of small molecule anticancer drugs were natural products or derived from natural products.
Unlike antibodies, small molecule therapeutics can be targeted with both intracellularly or extracellularly. For example, when a tumor reaches 1-2 mm in diameter, they begin to develop their own vasculature to provide enough nutrients for the tumor to continue to grow. Small molecule inhibitors can target vascular endothelial growth factor (VEGF), which is expressed on the cell surface of cells and promotes vascularization. Monoclonal antibodies (mAbs) act directly on VEGF, blocking the receptor to prevent vascularization. Small molecules acting as tyrosine kinase inhibitors act internally, blocking the downstream signaling of the receptor within the cell.
Antibody-based treatments
Over the past 20 years, antibody-based treatment has been one of the most successful strategies for treating cancer. As of 2012, there were at least 12 FDA approved monoclonal antibodies for the treatment of solid tumor and blood cancers. Unlike small molecules that can effect change intracellularly or extracellularly, antibody based treatment are based on cell surface antigens. Based on astounding work describing cancer cells, a detailed understanding of the cell surface receptors of cancer cells is growing, and aided by bioinformatics, could lead to a full picture of the "cancer surface-ome". Cancer cells often have antigens that are mutated or overexpressed compared to normal cells, creating ideal targets for antibody fragments. Selective expression in cancer cells compared to normal cells, which is sometimes considered a "magic bullet", has not yet been found.
The killing of tumor cells is done through direct action of the antibody such as blocking a growth-factor receptor, affecting the tumor vasculature, recruiting immune killer cells, or payload delivery (antibody-drug conjugates described below).
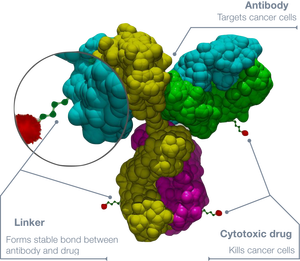
Antibody-drug conjugates
Antibody-drug conjugates (ADCs) are idealized as brining together the best of antibody and small molecule-based treatments. Antibodies are linked with a small molecule payload and serve as guided missiles to deliver cytotoxic drugs. While mAbs can precisely target a desired antigen, they can lack the therapeutic punching power to stop the cancer cells. Cytotoxic drugs, obviously, require precise local concentrations. By leveraging the guidance system of mAbs and the cytotoxicity of small molecules, many ADCs have been created with The success of ADCs is dependent on the target antigen, antibody, linker and payload. As of 2017, four ADCs have been FDA-approved for the treatment of cancer.
Biotechnology of drug compounds
As of 2016, there are at least 49 FDA-approved nanomedicines mostly consisting of polymers and liposomes. These drugs essentially functionalize the existing drug to improve the therapeutic in many possible ways, including increasing stability, increasing circulation time, reducing toxicity, and controlled delivery. The most common method is PEGylation, essentially a covalent linking of polyethylene glycol to the drug of choice (e.g. small molecule or antibody fragment). The other predominant method is lipsomes, which were the first nanomedicine in clinical trials, where self-assembling lipids contain the drug of choice either within the aqueous inner core or hydrophobic membrane. While polymers and liposomes, or a combination thereof, are the most common, the more recently developed protein-based and inorganic-based (read gold) nanoparticles have also seen FDA success and are a major component of drugs in clinical trials today.
Antibody-drug conjugates (ADCs) are idealized as brining together the best of antibody and small molecule-based treatments. Antibodies are linked with a small molecule payload and serve as guided missiles to deliver cytotoxic drugs. While mAbs can precisely target a desired antigen, they can lack the therapeutic punching power to stop the cancer cells. Cytotoxic drugs, obviously, require precise local concentrations. By leveraging the guidance system of mAbs and the cytotoxicity of small molecules, many ADCs have been created with The success of ADCs is dependent on the target antigen, antibody, linker and payload. As of 2017, four ADCs have been FDA-approved for the treatment of cancer.
Biotechnology of drug compounds
As of 2016, there are at least 49 FDA-approved nanomedicines mostly consisting of polymers and liposomes. These drugs essentially functionalize the existing drug to improve the therapeutic in many possible ways, including increasing stability, increasing circulation time, reducing toxicity, and controlled delivery. The most common method is PEGylation, essentially a covalent linking of polyethylene glycol to the drug of choice (e.g. small molecule or antibody fragment). The other predominant method is lipsomes, which were the first nanomedicine in clinical trials, where self-assembling lipids contain the drug of choice either within the aqueous inner core or hydrophobic membrane. While polymers and liposomes, or a combination thereof, are the most common, the more recently developed protein-based and inorganic-based (read gold) nanoparticles have also seen FDA success and are a major component of drugs in clinical trials today.
