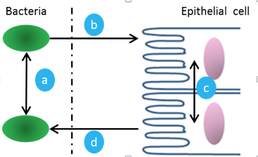
Bacteria communicate with each other through a process known as quorum sensing (QS), which uses signaling molecules, like the ‘universal’ signaling molecule autoinducer-2 (AI-2), to coordinate actions such as virulence and pathogenicity. Interkingdom cell-to-cell signaling involves small molecules, such as hormones that are produced by eukaryotes and hormone-like chemicals that are produced by bacteria. Through next-generation RNA sequencing and bioinformatics, our work indicated for the first time that quorum sensing impacts the behavior of human intestinal epithelial cells (IECs) through the ‘universal’ QS molecule AI-2 (mBio 2015). We found that E. coli secretions have an inflammatory effect in epithelial cells, and that specifically IECs ‘listen in’ on bacterial communication mediated by AI-2. As the levels of the AI-2 have been shown to affect the bacterial composition in the microbiome, further exploration could help clarify the mechanisms behind dysfunctional immune responses such as Crohn’s disease.
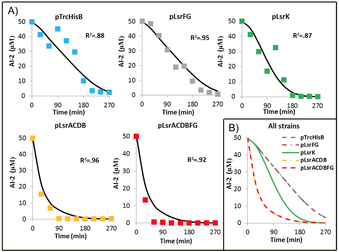
After determining the effects of AI-2 on epithelial cells, I genetically engineered a bacterium that disrupts bacterial communication by the rapid consumption of AI-2. This has numerous applications, most notably as an alternative to antibiotics as a means to control virulence and pathogenicity. A new approach to fight bacterial infections is to interfere with QS molecules, instead of killing the bacteria directly, thereby stopping pathogenic behaviors without causing resistance. Through a model-based design, we developed a suite of well-characterized controller cells that have elucidated the kinetics behind the signal transduction of the quorum sensing molecule AI-2. We demonstrated the clinically relevant applications of quorum interference in modulating expressions such as biofilms through these cells (Met. Eng. 2015). Using harmless probiotic bacteria to interfere with communication molecules provides a potent tool to stop bacterial infections without causing a resistance mechanism.
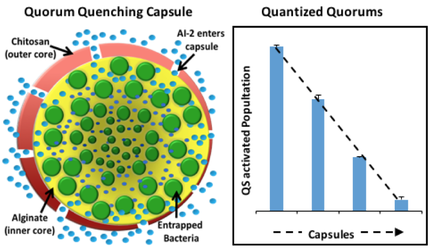
Based on the mathematical model we developed, I designed a bacterium with the highest rate of AI-2 uptake to manipulate QS behaviors from inside a biocompatible capsule (B&B, 2016). In my prior work, we showed that these controller cells could uptake AI-2 when interacting directly with the population. Useful in many infections, these cells are retained within the capsule, while AI-2 is small enough to freely diffuse into the capsule and be consumed. By minimally interacting with the cell population, the device was not only able to quench QS, but could also tune the phenotypes of QS subpopulations. There would be tremendous advantages in controlling the signal intensity so that some cells could make one designed protein, and some cells could make another, thereby generating ‘quantized quorums’, a concept advanced in a previous study (ISME 2016). This was a major leap forward from our work published in the ISME journal, where quantized quorums were generated by knocking out the luxS gene and adding metabolites. Now, we could guide one cell subpopulation to produce a product and another subpopulation to produce another, which is the major advantage of microbial consortia. Following up on this work, I described how discrete levels of molecules like AI-2 could be used to connect different cell populations together on a microfabricated chip as a ‘bioproduction breadboard’ (cover, Curr. Opin. 2016). By separating the populations but linking and coordinating them with discrete levels of AI-2, we could create microbial communities with balanced growth populations. This allows de novo biological pathways to be produced by leveraging the specialization of each cell type.
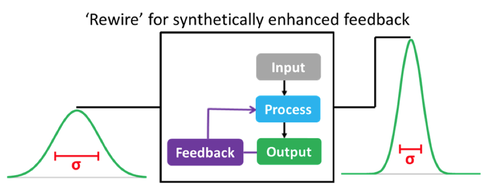
I developed a protein expression platform that autonomously redirects resources from growth and production to protein production (ACS Syn Bio, 2016). Typically, inducing agents are used to redirect these resources, but they are often prohibitively expensive on the industrial scale, require external monitoring to add at the ‘right time’, and can be thrown off task by environmental perturbations. We illustrated that by manipulating the QS circuitry, coordination and homogeneity was maximized in cell coordination, ultimately increasing protein production and reliability, without the use of inducers. This work is a significant step in population engineering for ‘programing’ cell performance where the intended modifications enable cells to stay ‘on task’ in the midst of changing environments. This will decrease costs and allow the production of biologics that were previously too expensive to make.
